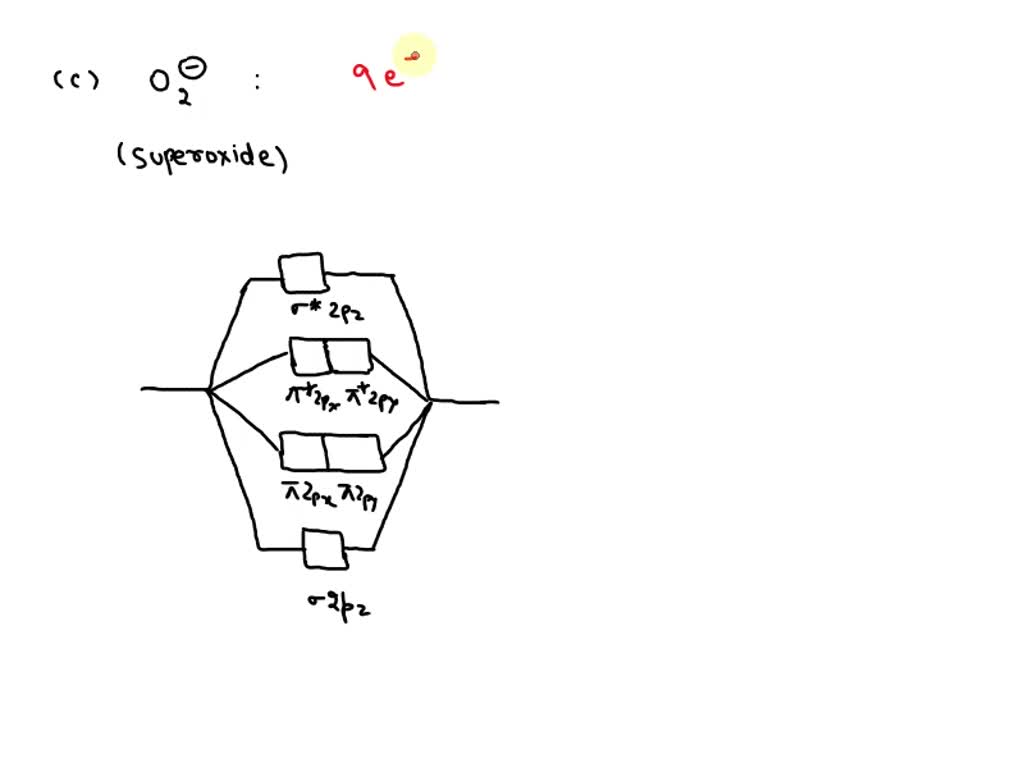
compare the relative stabilities of O2- and N2+and comment on their magnetic behaviour - Chemistry - Chemical Bonding and Molecular Structure - 13191415 | Meritnation.com

use molecular orbital theory to explain the magnetic behaviour and bond order of O2 Negative and O2+ molecules - Chemistry - Chemical Bonding and Molecular Structure - 13154833 | Meritnation.com
Why is oxygen paramagnetic in nature and sulphur diamagnetic even though both belong to the same group? - Quora

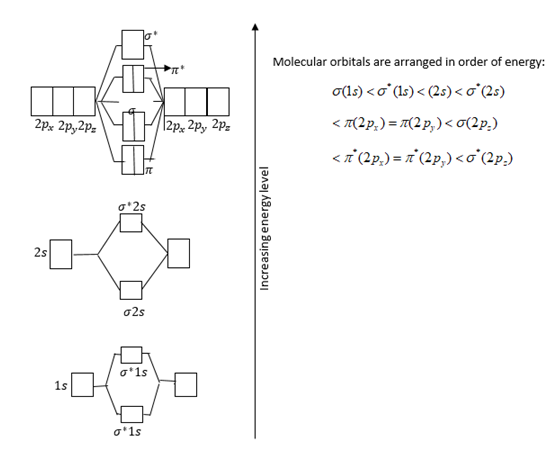
Molecular Orbital Theory || MOT || BMOs and ABMOs|| Bond Order || Magnetic Behaviour|| H2 Formation - YouTube
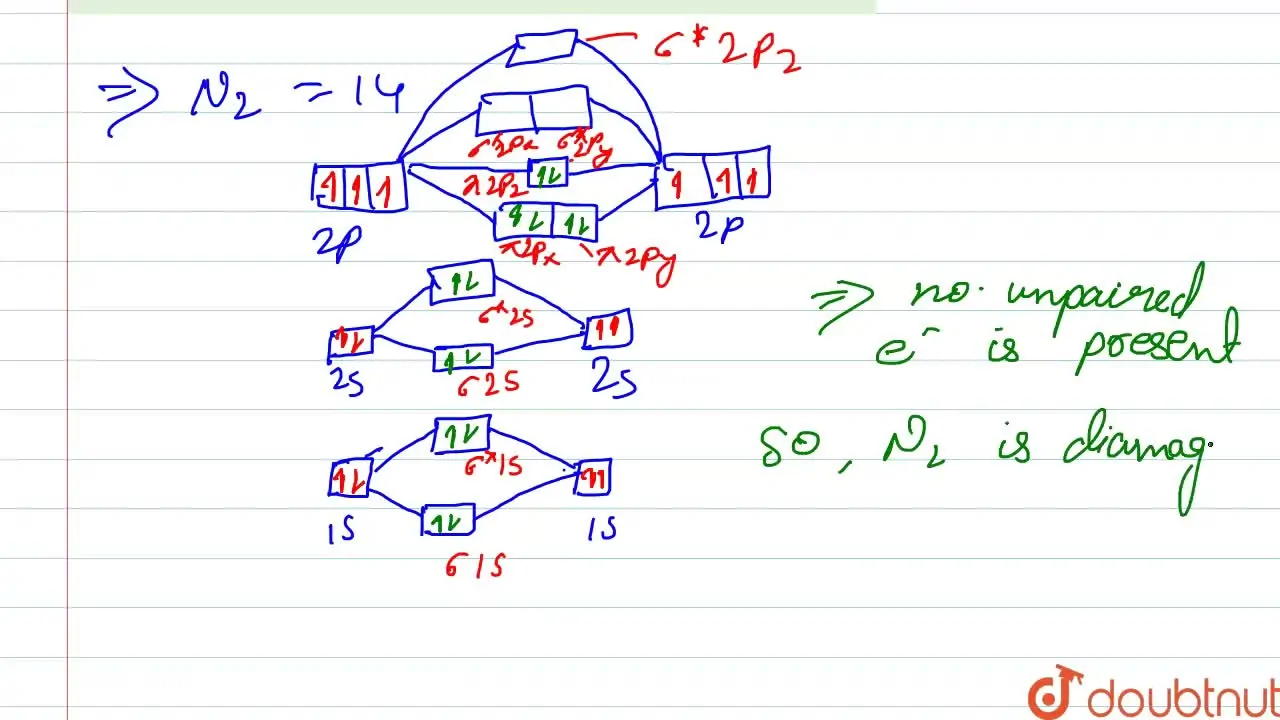
57.: In which of the following ionisation processes, the bond order has increased and the magnetic behaviour has changed (a) NO to NO+ (b) O2 to O2+ (c) N2 to N2+ (d)

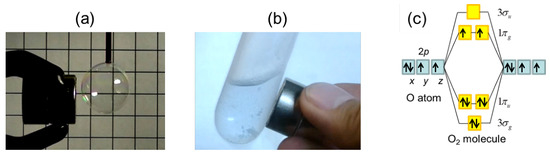
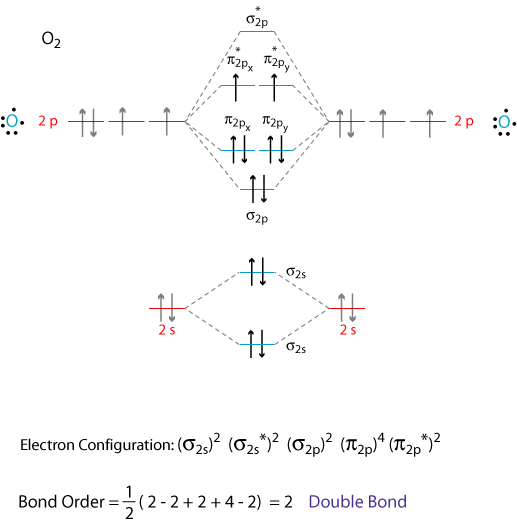
Schematic of the 'O2' molecular orbital diagram. The figure explains... | Download Scientific Diagram
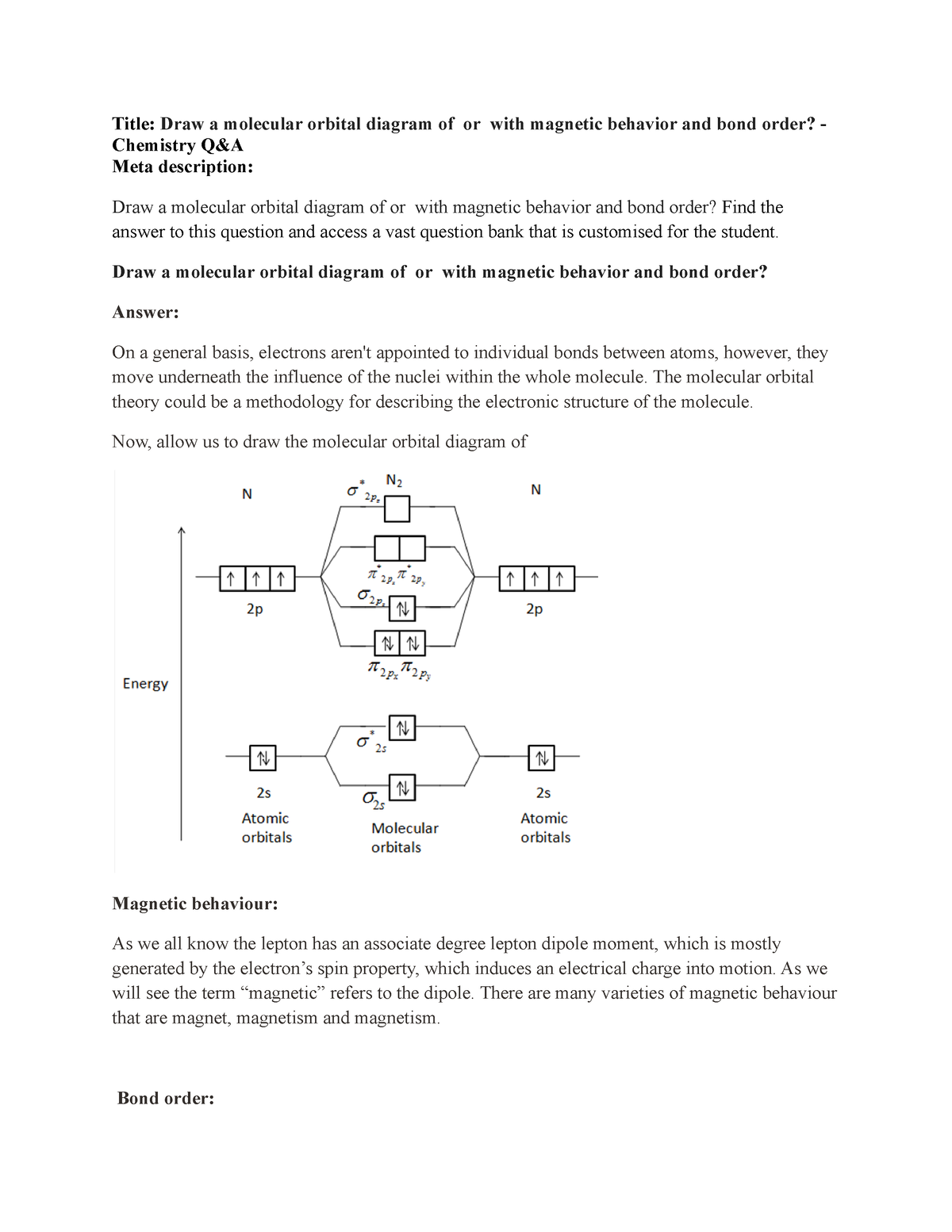
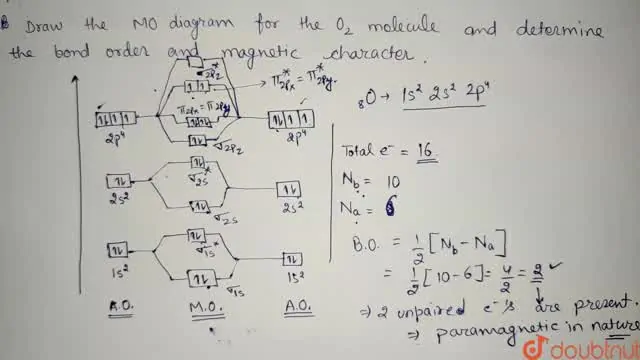
22-Draw a molecular orbital diagram of ${N 2}$ or ${O 2}$ with magnetic behavior and bond order - Studocu